- 2.57 MB
- 2022-04-29 14:35:22 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
'[理学]《聚合反应原理专论》chapter4中科大研究生教学课件
4-1Introduction1.Explanationofterms(1)SFRP:Stablefreeradicalpolymerization,nitroxide-mediatedradicalpolymerization(NMP)isefficientoneinSFRP(2)ATRP:Atomtransferradicalpolymerization,itisthemostsuccessfuloneinmetal-catalyzedlivingfreeradicalpolymerizations(3)RAFT:Reversibleaddition-fragmentationtransfer
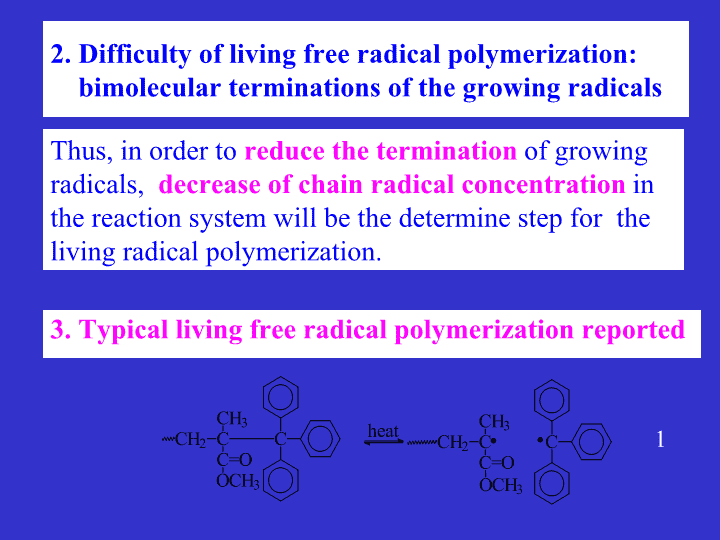
2.Difficultyoflivingfreeradicalpolymerization:
bimolecularterminationsofthegrowingradicals3.TypicallivingfreeradicalpolymerizationreportedThus,inordertoreducetheterminationofgrowingradicals,decreaseofchainradicalconcentrationinthereactionsystemwillbethedeterminestepforthelivingradicalpolymerization.1
4.Briefhistoryofmetal-catalyzedlivingradicalpolymerization(1)Atom-transferradicaladdition(ATRA)reactionsinorganicsynthesis
(2)ThepolymerizationviaATRAreactionIfthecarbon-halogenbondintheadductissuccessivelyactivatedbythemetal-complex,acontrolledradicalpolymerizationwillsimilarlyproceedviathemetal-assistedbythemetalrepetitiveactivationandformationofthecarbon-halogenbondatthepolymerchainend.
Itcancontrolthepolymerizationsincludingmethacrylates,acrylatesandstyrene.b.Eachmoleculeoforganichalideproducesonepolymerchain.
(3)componentsMonomerInitiators,R-X,SO2-XCatalystsLigandsSolventsAdditive
4-2Designoftheinitiatingsystem1.ThecompositionofinitiationsystemThechoiceofthemetalsandtheinitiatorsaccordingtothemonomerstructuresiscrucialforcontrollingradicalpolymerization.Therateandcontrolofpolymerizationcanalsobeincreasedbyadditionofsomeadditivesorbychangingsolvent.MnXnLm
2.Transition-metalcatalysts(1)Functionoftransition-metalcomplexInducereversibleactivation(homolyticcleavage)ofadormantcarbon-halogenbondatapolymerterminalviaaone-electronredoxreactionofmetalcenter
b.Theloweroxidationstateofthemetalcentercanestablishanextremelylowconcentrationoftheradicalspeciesaswellasafastreversiblereactionwiththehalogen
(2)Rutheniuma.Amongthetransition-metalcomplexes,onecomplexusedincontrolledradicalpolymerizationisRuCl2(PPh3)3etc.AmongthevariousoxidationstateofRucomplexes,thedivalentformwithphosphineligandshaseffectivelybeenemployedforthemetal-catalyzedlivingradicalpolymerization.MitsuoSawamoto(澤本光男)KyotoUniversity(京都大学)
b.Thecatalystsusedinthelivingradicalpolymerization
c.OneexampleofRucomplexusedinlivingradicalpolymerizationMWcanbecontrolledbythemolarratioofmonomertoinitiator,andconversion.RelativenarrowerMWD(Mw/Mn~1.3).
WhenusingCHCl2COPhandAl(O-i-Pr)3inplaceofCCl4andMeAl(ODBP)2,resultingPMMADoesthispolymerizationfollowfreeradicalpathway:ThepolymerizationcanbeinhibitedbyTEMPO,DPPH.H2OandCH3OHdidnotaffectthepolymerization.Eachmacromoleculehasonehalogenatomandcanbeusedasmacroinitiator.
Wheniodoinitiatorwasused,PolyStwithPDI=1.2wasobtained.ThePPh3-basedRu(II)hydrideismoreactivethanthechloride,inducingfasterpolymerization,andstillactiveat30oCtogivepolymerwithPDI~1.2.
ForRuwithanionicphosphineligandwhichissolubleinwaterandmethanolHomogeneouslivingfreeradicalpolymerizationinmethanol,noprotectionofhydroxygroupisneeded.
Foraseriesofthe18-electronhalf-metallocene-typeRucomplexesRu-5leadstothefastestlivingradicalpolymerizationofMMA,andadditivessuchasaluminumalkoxidesarenotnecessary,butadditionofamines,suchasn-Bu2NHcandramaticallyincreasetheratetocompletethepolymerizationin5hat100oCwithoutbroadeningtheMWDs.Ru-6isenablelivingradicalpolymerizationofthreetypeofmonomers,MMA,St,MA,inconjunctionwithachlorineinitiatorandAl(O-I-Pr)3.ThepolymerswithPDI=1.1-1.2forallmonomerswereobtainedintolueneat80oC.
Thecatalyticactivityincreasesinthefollowingorder:Ru-4<Ru-5<Ru-6Thelowertheredoxpotentialofthecomplex,thefasterthepolymerization.ComparisonofRu-6withRu-7Ru-7ismoreactivethanRu-6becausetheformerhasavacantsitethatcaninteractwithahalogenatapolymerterminalwithoutreleaseofphosphineligand.
Ru-carbenecomplexes(Ru-8)canmediatelivingradicalpolymerizationofMMAandSttoaffordcontrolledpolymerswithPDI~1.2.Ru-9carriesabromoisobutyrategroup,andcannotonlyinitiatebutalsocatalyzelivingradicalpolymerizationofMMAwithoutinitiator.Itisalsoactiveinring-openingmetathesispolymerizationof1,5-cyclooctadiene(COD).Ru-8Ru-9Ru-9
(3)Irona.Irontakesvariousoxidationstates.Amongthem,Fe(II),Fe(I)andFe(0)havebeenreportedtobeactiveinKharaschadditionreactions.Formetal-catalyzedlivingradicalpolymerizations,severalFe(II)andFe(I)complexeshavebeenprovedmoreactivethantheRu(II)counterpart.ThetypicalFe-basedcatalystsareasfollows:
b.ThefirstexampleofFecomplexusedinthelivingradicalpolymerizationisFe-1ThepolymerizationrateisfasterthanRuCl2(PPh3)3PMMAwithPDI=1.1-1.3.
c.FastlivingradicalpolymerizationofMMAandStusingFecomplexwithimidazoleligandPolymerizationsofMMAandStgivethepolymerswithPDI=1.1-1.3.AdditionofFeCl3slowedthepolymerization,butnarrowedMWDs(PDI=1.1).
d.TheuseofCporCp*-basedFecomplexeswillinducelivingradicalpolymerization(a)PStwithverynarrowMWD(PDI=1.1)andcontrolledMWs(b)Theratewillincreasewiththeuseofthecorrespondentbromide,whileMWDwasnarrowedbyusingsubstitutedCp*,suchasFe-4.(c)AfasterandcontrolledpolymerizationwaspossiblebyusingFe-5andFe-6inabsenceofmetalalkoxides.Fe-3Fe-4Fe-5Fe-6
e.Mixturesofiron(II)halides(FeBr2andFeCl2)andligandswerealsoemployedforlivingradicalpolymerizationAttention
ReverseATRPFirstorderkineticsThemolecularweightincreasedlinearlywithconversionandPDIswerequitelow(Mw/Mn<1.3).Theinitiatorefficiencyinbulkpolymerizationislowerthaninsolution,duetoalargerportionofterminationinbulkpolymerization.Aninhibitionperiodwasobservedattheinitialstageofpolymerization.
(4)CopperCoppercatalystshavebeenextensivelyusedformetal-catalyzedlivingradicalpolymerization.TheinitiatingsystemisamixtureofCu(I)BrorCu(I)Clandanitrogen-basedligandorinselectedcomplexesbyisolatedcomplexesThenitrogenligandcanbeclassifiedintobidentate,tridentate,quadridentateandhexadentate.王锦山,KrzysztofMatyjaszewskiDepartmentofChemistry,CarnegieMellonUniversity
a.BidentatecompoundsThefirstsystemconsistedofCuClandL-1,1-phenylethylchlorideasinitiator.Thepolymer(St)withMW=100,000;PDI<1.5,ItisalsoeffectivetoMA(PDI~1.1)andMMA(PDI~1.3).Forimprovingitssolubilityinthesolvent,longchainalkylgroupson4,4’-positionsofbipyridinesuchasL-2,L-3,andL-4,weresynthesized.L-3L-2L-5L-1L-4L-6L-7L-8
Thepolymerizationinhomogeneoussystemismuchfasterthantheheterogeneoussystem,andgivethePStverynarrowMWDs(PDI=1.05).If10%ofDMFwasaddedintothesystem,thePStwithbroadMWD(PDI=1.4-1.8)wasobtained.L-9L-10L-11L-12L-13L-14L-15L-16L-17
ThedifficultyinisolationandclarificationofCu(I)-bpycomplexeswasovercomebyusingsi-containingBpy.WhenitmixedwithanequimolaramountofCuBr,anioniccomplexwasgenerated.ThecontrolleddegreeoftheradicalpolymerizationissimilarwithCuBr/Bpysystem.Aflluoroalkyl-substitutedbpywasusedinthesupercriticalCO2forthepolymerizationoffluorinatedacrylatesandmethacrylates.L-6L-7
Cu(II)complexwithbpy-basedligandsuchasL-4,iseffectiveinthecontrolledradicalpolymerizationsinthepresenceofAl(O-i-Pr)3,whichmostprobablyservesasareductionagentofCu(II)intoCu(I).L-8wasalsousedforCuCl,CuBr,andCu(0)inthecontrolledradicalpolymerizationofStandMMA.L-8(R=Ph)withCuClindimethoxybenzenesolutionaffordedbettercontrolledpolymerizationthanL-4didunderthesamecondition.L-4L-8
Aseriesofschiff-baseorpyridium-typebidentateligandshasefficientlyusedforhomogeneouspolymerizationofMMAderivatives(PDI=1.1-1.2)withCuBrandbromideinitiatorinnonpolarsolventsuchastoluene,p-xyleneThesolubilityoftheligandsdependsonthealkylsubstituentsandhomogeneitycanbeachievedwithalkylchainslongerthann-Buat90oC.TherateincreasesfromEtton-Pr,noincreasewasobservedwithlongerchainlength.Slowerandlesscontrolledradicalpolymerizationwasobservedwhenbranchingwasintroducedat-positionofthesidechain,suchasL-10.L-10L-9
ThepolymerizationofMMAwasperformedinxyleneat90oCwithabromineinitiator.Thetacticityofthepolymerobtainedisnotdifferentfromthatinconventionalpolymerization,butMWiscontrolled.L-11Thepossiblecontrolofstereochemistrywasinvestigatedwiththeuseofchiralschiff-basesL-11(R=Ph,Cy).
TheremovalofthecatalystisimportantforapplicationWhenL-13wasusedasligandinthepolymerizationofMMAwithCuBr,thecatalystcanremovedfromthepolymerobtainedcompletely.DiaminecompoundssuchasL-15coordinatetocopperspecies,buttheiruseinpolymerizationofMMA,MAandStresultsinslowpolymerization,andbroadMWDs(PDI=1.3-2.5).L-13L-15
b.TridentatenitrogenligandsForm1:1complexwithcopperwhichiscontrasttobidentateligandsthatformtetrahedralioniccomplexes.Well-controlledradicalpolymersofStandMAareobtainedwithasubstitutedterpyridine(L-18).Whereastheunsubstitutedderivativesinduceduncontrolledpolymerization.L-19L-20L-21L-22L-25L-24L-18L-23
Atridentateligandwithtwopyridinesandoneamine(L-19)givesnarrowMWDsforSt,MMAandMA(PDI=1.1-1.4).MnvalueswereslightlyhigherthanthecalculatedvaluesforPMMA.AnotherligandL-20waseffectiveforStandMA,L-21gavenarrowMWDsofSt.L-22canbeemployedforcontrolledradicalpolymerizationofMMA(PDI<1.3).L-23iseffectiveforMMAandSt(PDI<1.3).L-19L-20L-21L-22L-23
Linearandcyclictriamines,suchasL-24andL-25canbeusedinthelivingradicalpolymerizationofthreetypesofmonomers,MMA,MA,St,andgivetherelativelynarrowMWDs(PDI=1.1-1.4).ThepolymerizationsofStandMAarefasterthanthosewithBpy.L-24isusefulinlivingradicalpolymerizationofMMAunderfluorousbiphasicconditions.Thereactionmixtureswerehomogeneous.L-24L-25L-26
c.Ligandswith4nitrogen-basedcoordinatingsitesL-27,CuBrandCu(0),bromideasinitiatorforcontrolledradicalpolymerizationofMMA(PDI=1.1-1.4).L-28inducedfastpolymerizationofMMA,MAandSt.L-29affordedbroadMWD,butfastpolymerizationofdimethylacrylamide.L-30and31areeffectiveinthecontrolledradicalpolymerizationofStandacrylates.Butveryfastcontrolledradicalpolymerizationofacrylatesproceedsto80%conversionevenat22℃.L-28L-29L-30L-31L-27
d.CoppersaltsThesaltsseemtoaccerleratepolymerizationduetotheformationofunbridgedmonomericandactiveCu(I)species,butinorganicsolvent,Cu(I)Xgenerallyformsbridgeddimericcomplexes.
CuOTfinducedfastpolymerizationofMMAandSt.CuPF6/L-24inducedmuchfasterpolymerizationofMA,theKpis40timesthanthatofCuBrwithcontrolledMWbutbroadMWD(MWDs=1.4-1.6).Cu(OAc)/L-4ledtofastpolymerizationofSt,butbroadMWDs.Cu(II)inducedcontrolledpolymerizationofMMAandn-butylmethacrylate,theratesincreasedintheorder:CuO<CuS<Cu2Se<CuCl<Cu2Tewithsulfonylchlorideasinitiator.
(5)NickelAmongthevariousoxidationstatesofNickel(0-IV),Ni(II)andNi(0)arethemoststableNi-1wassuccessfullyemployedforthelivingradicalpolymerizationofmethacrylateswithCCl4or(CH3)C(CO2C2H5)2Brasaninitiator.Thepolymerizationproceededhomogeneouslyandreached80%conversioninabout1dayat80oCtogivethepolymerwithnarrowMWDs(PDI=1.1-1.2).Ni-2/bromide/Al(O-I-Pr)3inducestheLFRPofMMAintolueneat60and80oC.Itschlorideisnoteffectiveinthepolymerization.Ni-3/bromidecanbeemployedforMMA,MAinawiderangeoftemperature(60-120oC),at120oC90%conversionin2.5h.
(6)PalladiumPd(0)andPd(II)arestable.Pd-1andPd-2wereusedinthelivingradicalpolymerizationofMMAwithCCl4asinitiatorintolueneat70oC.70-80%conversionin24h.MWDwasbroader(PDI=1.8).PolymerizationofStandMAisnotcontrolled.Pd-1Pd-2
(7)RhodiumItcaninducefastpolymerizationofMMAwithCCl4orCHCl2COPhasinitiatorinTHForamixtureofTHFandwaterat60oC,90%conversionin4h,givethepolymerwithMWDs=1.3-1.7.Rh-1(x=Cl)/sulfonylchloridewasusedinthepolymerizationofStinbulkandat130oC,andpolymerwithMWDs=1.7.Rh-1/bromideinducedthepolymerizationofMMA,togiveMWDs=1.3Rh-2/CCl4wasusedforMMAandStbulkpolymerizationat60oC,90%conversionin14h,PMMA(1.43)vsPSt(2.08).Rh-1Rh-2
(8)ImmobilizedcatalystsInordertosolvetheproblemthatthecatalystisverydifficulttobeseparatedfromthepolymersobtained,theimmobilizedcatalystshavebeendeveloped.ThesolidsupportsaresilicagelandPStparticlesSL-1/CuBrwasusedinthebulkpolymerizationofSt,resultedinpolymersofuncontrolledmolecularweightsandbroadMWDs(Mw/Mn=2-10).SL-1
Organicpolymer-supportedligandsoncrosslinkedpolystyreneSL-2andSL-3/CuBrwasusedinthepolymerizationsofacrylatesandmethacrylatesandstyrene,resultedintheuncontrolledradicalpolymerization.SL-4/CuBr2/CuBrinducedthepolymerizationofMAtogivetheMWclosertocalculatedvalueMWD=1.6-2.0.SL-2SL-4SL-3
SL-5inducedcontrolledradicalpolymerizationofMMA,resultinginnarrowerMWDs=1.5-1.6,theMWishigherthanthecalculatedMW.BlockcopolymerizationandthereusedthecatalysthavebeenalsoachievedSL-6/Ru-1inducedthepolymerizationofMMAwiththeMWclosertothecalculatedvalue,MWD=1.5-1.7,Blockcopolymerizationandreusedarepossible.SiO2/L-28/CuBrwasusedinpolymerizationofMMAtogivethepolymerwithMWD=1.3.TherecycledcatalystgivesnarrowMWD=1.2andtheMnagreeswellwiththecalculatedvalues.L-28SL-5SL-6
Summaryandoutlook1.Somecatalyticsystemsreportedmayleadnotonlyfreeradicalprocess,butalsoionicand/orcoordinationpolymerization.2.SomeIodine-basedsystemmayredox-initiatepolymerizationandattaincontrolduetoIdegenerativetransferprocess.3.Thelateandmiddletransitionmetalcomplexes,suchasCu,FeandRu,aremostefficientcatalystsinATRP.Theactivityandselectivityarestronglyliganddependence.Itisinterestingtoexplorelanthanidesaspotentialcatalystsduetotheflexibilityofcoordinationsphereandlargerangeofredoxpotentials.
4.Idealcatalystshouldbe:(d).Easilytunableactivationrateconstantstomeettherequirementofspecificmonomers,forexample:ForStandMA,K>10-8;foracrylamide,K>10-7;ForVAandVC,K>10-5.(a)Highlyselectiveforatomtransfer.(b)Notparticipateinotherreaction.(c)Deactivateextremelyfastwithdiffusion-controlledrateconstant.
3.Initiator(1)Theimportanceoftheinitiatora.Providetheinitiatingradical.b.Thedormantpolymerchainendshouldbestableduringthepolymerization.c.Theinitiatorischosensothattheinitiationoccursfast,andisquantitative.Mostoftheinitiatorssuccessfullyemployedareorganichalideswithapotentiallyactivecarbon-halogenbond,whichcaneasilygeneratearadicalthroughelectronicandstericeffectsoftheirsubstituents.
(2)ThestructureofinitiatorCHnX4-nR1R2R3C-Xa.ForXgroupThereactivityofXgroupsincreasesinthefollowingorder:Cl<Br<I.ThestabilityoftheC-Xbonddecreasesinthesameorder,soClandBrhavebeenwidelyemployed.b.ForpolyhalogencompoundsTheymayactasmultifunctionalinitiators.c.Rgroup:(a)AtleastoneofR1,R2andR3isaconjugatedsubstitutedgroupsuchasallyl,aryl,carbonyl,andcyanogroupsetcforstabilizationofthegeneratedradicals.(b)Thecarbon-halogenbondintheinitiatorandattheendofthepolymershouldbesimilar.Forexample,CH3-CHBrPhinitiatesSttoform-CH2-CHBrPh.
a.HaloalkanesRu(I)/CCl4inducedlivingradicalpolymerizationofMMA.Mnincreasedlinearlywithconversion,MWDs=1.3.Tri-,di-ormonochloromethanescannotinducepolymerizationofMMA..IntheCu(I)Br/CCl4andStpolymerizationsystemat130℃,CCl4actedasbifunctionalinitiator.CCl4isatransferagentandatelomerinfreeradicalpolymerization.Anotherproblemisthatsuchpolyhalogencompoundsmayactasmulti-functionalinitiatortoformtelechelicorstarpolymers.Amongthesecompounds,HCCl3isnotgoodinitiatorintheRu-1basedsystemprobablyduetolowactivityorthehighredoxpotentialoftherutheniumcomplex1234567
b.AllylhalidesAllylradicalisrelativelystable.Allylchloride(bromide)/CuCl(orCuBr)/BpyinducedthelivingradicalpolymerizationofSt,andgavethepolymerwithMWDs(PDI=1.2-1.3).
c.HaloalkylbenzenesCompounds12-15wereexaminedextensivelyandprovedeffectively,especiallyforpolymerizationofStanditsderivatives.Amongthem,13canbeconsideredasunimerofthedormantC-XterminalofPSt,thustheyareparticularlysuitedforcontrollingpolymerizationofSt.WhenCuBr/L-4wasused,PStwithPDI=1.1wasobtained.12131415
Benzylichalidesfailedinthepolymerizationofmorereactivemonomers,suchasMMA.When1-phenylethylchloridewasemployedastheinitiatorforthepolymerizationofMMA,PMMAwithhigherMWsthanthetheoreticalvaluesandhighMWDs(Mw/Mn=1.5-1.8).WhenPh2CHClasinitiatorofMMApolymerization,well-controlledradicalpolymerizationwasrealized.
PolyhalogenatedbenzylichalideswereusedintheATRPofMMAcatalyzedbyRuCl2(PPh3)3/Al(OiPr)3WhenPh2CCl2wasusedasinitiator,PMMAwithverylowMWDwasobtained.PhCCl3ledtoabimodalMWDconsistingoftwonarrowlydistributedfractions,thehigherofwhichwasdoubletheMWoftheother.PhCHCl2hasalsousedinCu-basedATRPofStandMMA,providingtwo-directionalgrowthofthepolymericchains(Cu/bPy).Macromol.Chem.Phys.201,980–984(2000).
LivingradicalpolymerizationwithchloromethanesmolecularweightsandMWDsofpolymersobtainedwithchloromethane/RuCl2(PPh3)3/Al(OiPr)3initiatingsystemsintolueneat80oC:[MMA]0=2.0M;[initiator]0=20mM;[RuCI2(PPh3)3]0=10mM;[Al(OiPr)3]0=40mM;conversionforMWDsamples>90%.Tetrahedron,Vol.53,No.45,pp.15445-15457,1997.
d.HaloketonesRadicalformationisfavoredbyintroducinganelectron-withdrawingandconjugatingsubstituentinthe-positionofC-Xbond.Compounds16(or18)/Ru(II)[orNi(II)]caninducedlivingradicalpolymerizationofMMA.16(or18)/Ru-1gavethePMMAwithPDI=1.1-1.2,MWisingoodagreementwiththecalculatedvalue,theMWisproportionaltoconversion.Haloketonesaregenerallytooreactiveforthecopper-catalyzedhomogenoussystemsandresultinuncontrolledpolymerizations,probablyduetothereductionoftheelectrophilicradicalspeciesintoanionsbythehighlyactiveCu(I).161718
e.HaloestersHaloestershavebeensuccessfullyemployedwithawiderangeofmetalcomplexes,includingRu,Fe,Cu,Ni,etc.Alesselectron-withdrawingestergroupcanmoderatelyactivatetheC-Xbondanddoesnotmaketheresultingradicaltooelectrophilic.ThistypeofinitiatorcanbemoreversatileforvariousmonomersincludingSt,MMAandMAetc
Thehaloestersusedinthelivingradicalpolymerizationareasthefollows.19or20/Ru-1mediatedlivingfreeradicalpolymerizationofMMA.21and22aresuitableforthecontrolledradicalpolymerizationofacrylates,Standacrylamides.Bromidesarebettercontrolthanchloride.Compounds21or22/CuBr/BpywasusedinthepolymerizationofMA,thePMAwithPDI=1.2wasobtained.21,22and23aregenerallylesssuitableforthelivingradicalpolymerizationofMMAduetothelowreactivityofC-Xbondscomparedwiththatofpolymerterminal.1920212223
Compound24ismoreversatileforvariousmonomers,suchasMA,MMAandSt,variousmetalcomplexesincludingRu,Fe,Cu,andNiduetounimermodelofpoly(methacrylate)s.Compounds27and28inducecontrolledpolymerizationofbothMMAandStinthepresenceofCuBr/Bpy.Compound25hashigherreactivitysimilarwith24.Compound26generatedastableradicalrapidly,canbeemployedforLRPofMMAwith(PPh3)3RuCl2toaffordnarrowMWDs,butslightlyhigherMnthanthecalculatedvalues.26/CuBr/L-4cancontrolofMWofPMMA.NopolymerizationoccurredwithCH(CO2Et)2Br.2425262728
f.HaloamidesCompounds29and30canbegoodinitiatorsforacrylamides.TheywereemployedfortheRu-catalyzedpolymerizationofN,N’-dimethylacrylamide(DMMA)togivecontrolledMWsandbroadMWDs(PDI=1.6).2930
g.HalonitrileCompound31(X=Cl,Br)unimersofhalogen-cappeddormantpoly(acrylonitrile),arespeciallyemployedforthepolymerizationofacrylonitrilewithcopperhalides.Thestrongelectron-withdrawingcyanogroupfacilitatestheformationofinitiatingradical,andmayalsoemployedforothermonomerssuchasStwithCuCl/bpy,MMAwithFecatalyst.31
h.SulfonylhalidesComparisonwithCarbon-centeredradicals,sulfonylhalidesparticularlyarenesulfonylhalidescangenerateinitiationradicalmuchfasterwiththeaidofametalcomplex,andaddefficientlytoolefinswithlittledimerizationofsulfonylradicals.Anotherfeatureisthatthereislittleeffectofsubstituentsontherateofadditiontoanolefin.ThesepropertiesmakesulfonylhalidesanefficientanduniversalseriesofinitiatorsforLFRPofvariousmonomersincludingmethacrylates,acrylatesandstyrenes.3233343536VirgilPercecDepartmentofChemistry,UniversityofPennsylvania(DepartmentofMacromolecularScience,CaseWesternReserveUniversity,1999)
ControlledMWandrelativelynarrowMWDs(Mw/Mn=1.2-1.4)InhomogenouspolymerizationsystemofSt,MMAandnBAwithCuCl/L-4/32,narrowMWDs(Mw/Mn=1.1-1.3)Kiapp:104kpforpolymerizationofStandMMA;103kpfornBA;102kpforMA.Theuseofarenesulfonylhalides/RucomplexcaninduceLFRPofMMA,Mw/Mn=1.2-1.5,Ieff~0.4,soMnvaluesarehigherthanthecalculatedvalues.
OverviewofDifferentStructuralParametersontheActivationRateConstantsMacromolecules2007,40,1857-1863.
ATRPequilibriumconstantsKATRPforvariousN-basedligandswiththeinitiatorEtBriBinthepresenceofCu(I)BrinMeCNat22°CJ.Am.Chem.Soc.2008,130,10702–10713
SummaryforinitiationImportantdemandsforATRPinitiationsystem:2.Generalconsiderationsfortheinitiatorchoice:Initiationshouldbefastincomparisonwithpropagationrate.Theprobabilityofsidereactionsshouldbeminimized.Thestabilizinggroupsorderintheinitiatorisroughly:CN>C(O)R>C(O)OR>Ph>Cl>Me.Multiplefunctionalgroupsmayincreasetheactivityofthealkylhalide.
GeneralorderofbondstrengthinthealkylhalidesisR-Cl>R-Br>R-I.R-Iislightsensitive,andcanbecleavedheterolytically,therearepotentialcomplicationsoftheATRPprocessbydegenerativetransfer.UseofCuClcangetbettercontrolofpolymerizationincomparisonwithuseofCuBr.Halogenexchangecansometimesbeusedtoobtainbetterpolymerizationcontrol,forexample,
ExchangeofBrandClforbettercontrollingpolymerizationMacromolecules1998,31,6836.FasterSlower
4.Themethodororderofreagentadditioncanbecrucial.Forexample,Thediethyl2-bromomalonate/CuBrsysteminitiatestheATRPofSt,andpolymerizationwaswellcontrolledwhencatalystwasaddedslowlyintotheinitiator/monomersolution.Thisavoidedthepotentialreductionofthemalonylradicalbythecopper(1)species.3.SuccessfulinitiationinATRPcandependstronglyonthechoiceofcatalyst.Forexample:2-bromoisobutyrophenoneinitiatesthecontrolledpolymerizationcatalyzedbyRuorNicomplexes,buthasnotbeensuccessfullyusedinCu-mediatedATRP,sincethecoppercatalystshavelowerpotentials.
4.MonomerMostofthemareconjugatedmonomerssuchasacrylates,methacrylates,styrene,acrylonitrile,acrylamidesetc.Howeverlessconjugatedmonomerssuchasvinylchloride,vinylacetateandethylenearestilldifficulttopolymerizeinacontrolledfashionbymetal-catalyzedpolymerization.Thismostprobablyduetothedifficultyinactivationoflessreactivecarbon-halogenbond.Metal-catalyzedlivingradicalpolymerizationhasallowedavarietyofvinylmonomerstobepolymerizedintowell–definedpolymersofcontrolledMW,andnarrowMWDs.
StructuresofthemonomersThethreetypeofmonomershavebeenextensivelystudied,andachievedbytheuseofvariousmetalcomplexesincludingRu,Fe,Cu,Ni,Pdetc.Methacryaltes,acrylates,andStyrenes
(1)StyreneInitiator:Monomers:Copper,iron,rutheniumandrheniumcatalyticsystem,Cuisusedascatalystinmajorityoftheresearchworks.Catalysts:
Ligands:PSt:rangeofMW:103-105;Lowerpolymerizationtemperatureforreducingthermalself-polymerization;Styreneswithelectron-withdrawingsubstituentspolymerizefaster.Solublecatalystsystem:CuBr/PMDETAorCuBr/dNbpySolvent:nonpolarsolventsarerecommended
(2)AcrylatesMonomer:Catalysts:Copper-,ruthenium-,andiron-basedsystem.Cuappearstobesuperioroverothertransitionmetalsinproducingwell-definedpolyacrylates.Thisispartiallyduetothefastdeactivationofthegrowingacrylicradicalsbythecuprichalides.
Initiator:a.PolyacrylateswithMWupto105andMw/Mn<1.1.b.Awiderangeofpolymerizationtemperaturesarepossibletoproducepolymerswithinareasonabletime,suchas,0.05mol%ofCuBr/Me6TRENascatalyst,PMAwithMn=12600andMw/Mn=1.10wasobtainedin1hatroomtemperature.c.AllylacrylatewassubjectedtoATRPconditionswithbpyordNbpyasligand,across-linkedreactionoccurred,evenat0oC.
(3)MethacrylatesMonomersCatalysts:Ru,Cu,Ni,Fe,Pd,Rhcatalyticsystem.
Initiatorsa.FacilepolymerizabilityofMMAandtherangeofavailablecatalystsisduetotherelativeeaseofactivationofdormantspeciesandthehighvalueoftheATRPequilibriumconstants.b.TypicalradicalconcentrationforthebulkandsolutioncontrolledATRPofMMAareestimatedtobebetween10-7-10-9M.c.MostpolymerizationsofMMAwerecarriedinsolutionat70-90oCinordertoreducetheconcentrationofpropagationradicals.d.MWofPMMA:1000to180,000.
(4)VinylpyridineOneofthedifficultiesinthepolymerizationisadecreaseofreactivityimposedbythecoordinationofmonomerwithmetalcomplex.Controlledradicalpolymerizationof4VPwasachievedbyaninitiatingsystemsuchasL-32,2-chloroethylbenzene/CuClin2-propanolat40oC.PDIofthepolymerobtainedis1.1-1.2.2VPcanbecontrolledradicalpolymerizationwithCl-PStasinitiatorCuCl/bpyinp-xyleneat140℃.BlockcopolymerwithPDI=1.1-1.2.L-32
(5)AcylonitrileAgoodcontrolofMWandMWDswasaccomplishedforthismonomerwithcompound31asinitiatorandCuX(Cl,Br)/bpyinethylenecarbonateat45-46oC.TheMniscontrolledupto30,000,keepingrelativelynarrowMWDs(PDI<1.5).31
(6)(Meth)acylamidesThecontrolledradicalpolymerizationofmethacrylamidewascarriedoutintolueneandat80oCwith29asinitiatorandRu-1/Al(O-i-Pr)3togivepolymersofcontrolledMWandrelativelybroadMWDs(PDI=1.6).Twosidereactions:theinactivationofthecatalystsbycomplexationofcopperbytheformingpolymers;displacementoftheterminalhalogenatombytheamidegroup.
HPMAAForHPMAA,CuBr/Me4Cyclam,inbutanol,PHPMAAwithMn=21300,Mw/Mn=1.38.Blockcopolymer,PBA-b-PHPMAAwithMn=34000,Mw/Mn=1.69.Cu-basedsystemsareapplicable,butpolymerizationstronglydependsontheinitiator,CuX,ligands,temperatureandsolvents,suchas,CuCl/Me6TREN,50%toluene,20oC,poly(N,N-dimethylacrylamide)withMn=8400,PDI=1.12.Blockcopolymers,PMA-b-PDMAAwithMn=4800,Mw/Mn=1.33.29
(Meth)acylicacidsATRPofAcrylicacidisnotsuccessfulduetothatacidmonomerscanpoisonthecatalystbycoordinatingtotransitionmetal.Protonatethenitrogencontainingligands.ChoiceofpHandcatalystarecrucial,TheoptimumpH=8-9;abalancebetweenthereducedpropagationrateathighpHandcompetingprotonationoftheligandatlowpH.
MiscellaneousMonomers
SummaryformonomersSeveralclassesofmonomershavenotbeensuccessfullypolymerizedbyATRPAcidicmonomersfailsincetheycanprotonateligandsandformthecorrespondingcarboxylatesalts.Halogenatedalkenes:vinylchloride,dichloroethene.Vinylesters:vinylacetateα-Alkenes:ethene,propene,isobutene.Theybelongtoaclassofmonomerswithverylowintrinsicreactivityinradicalpolymerizationandradicaladditionreactions,couldhaveverylowATRPequilibriumconstant.
5.additivesATRPistoleranttoavarietyoffunctionalgroups,suchaswater,aliphaticalcohols,polarcompounds.ButadditionofpyridineandPPh3intothepolymerizationsystemwillleadbigdecreaseofpolymerizationrateandincreaseofPDI.2.ATRPismoderatelysensitivetooxygen.ThepolymerizationofmethacrylatesinthepresenceofasmallamountofoxygenandCu(I)orCu(II)complexesyieldshighMWproductsandrelativelylowPDI.?
3.Metal-catalysislivingradicalpolymerizationareslowinmost
casesduetolowconcentrationofchainradicals.Apromising
solutionoftheinherentproblemistheuseofaddititives.Theseadditivesmostprobablycaneffectivelyreducethemetalspeciesinhigheroxidationstatesortoformmoreefficientcatalystsviacoordination.MetalalkoxidessuchasAl(O-i-Pr)3areemployedforRu,Fe,Ni,Rh,andCu-catalyzedpolymerizationandareeffectiveinincreaseinpolymerizationrateandnarrowingMWDsZerovalentmetalssuchasCu(0)andFe(0)effectivelyreduceCuBr2andFeBr3intoactiveCuBrandFeBr2respectively,todramaticallyincreasethepolymerizationrate.OtheradditivesarePhenol,benzoicacid.Forexample,
AdditionofbenzoicacidintoMMApolymerizationsystemwillincreasethepolymerizationrate,PDIalsoincreaseswithanincreaseinbenzoicacid/Curatio.ATRPofMMAusingvariousphenolsasadditivesresultsinthesmallincreaseofpolymerizationrate.
6.Solvents(1)Metal-catalysislivingradicalpolymerizationcanbecarriedoutinnonpolarandlesspolar,suchastoluene,xylene,benzeneetc.Tolueneisknownaschaintransferagent,buttheeffectofpotentialchaintransferagenthasnotbeenexaminedyet.(2)Polarsolventsproticsolventssuchasalcoholandwateralsocanbeusedinthesystem.Aproticpolarsolventsusedinthepolymerizationincludedimethoxybenzene(DMB),diphenylether(DPE),ethylenecarbonate,acetonitrile,DMFandacetone.MostofthemareemployedforCucatalystbecauseoftheirlowsolubility.Forwell-solubilizedRu(II),Ni(II)andFe(II)complexeswithphosphineorotherligandsarenotnecessary.InthehomogeneouspolymerizationofMMAwithphenysulfonylchloride/CuBr/L-9,therateincreasesintheorderxylene<DMB<DPE.
4-3Suspension,dispersionandemulsionpolymerizationTheproblemsinheterogeneousconditionsThepartitioningofthemetalspeciesintowater,whilethegrowingpolymerterminalexistsinorganicphase.Someundesirableinteractionamongmetalcatalysts,ligandsandadditivessuchasdispersantsorsurfactants.Theclassificationofthemetal-basedheterogeneoussystemintosuspension,dispersionandemulsionpolymerizationssimilartotheconventionalsystem.
2.VariousmetalcomplexesofRu,Fe,Cu,NiandPdareactive
intheradicalpolymerizationRu-1/organichalideinducedlivingradicalpolymerizationofMMAinmixtureoftolueneandwater,andyieldsthepolymerswithcontrolledMWandnarrowMWD(PDI=1.2).Whenthesystemisdispersedinwater,thepolymerizationstillcontrolled,butMWDsbecomesbroader(PDI=1.4).(b)TheCp-basedFecomplexprovedeffectiveinlivingradicalsuspensionpolymerizationsofacrylatesandSttogivenarrowMWD(PDI=1.1-1.2).Example:TheaqueousheterogeneouspolymerizationofhydrophobicMMA,MA,andStgavethecontrolledMWandnarrowMWDs.
4-4.MechanismofpolymerizationConfirmthefollows:ChainfreeradicalTerminalgroupofthepolymersFastequilibriumreactionLivingnature
1.Radicalscavengers2.EPRThepolymersandterminalgroupshavebeenanalyzedbymodernmassspectrometry,MALDI-TOF-MS,time-of-flightstaticsecondaryionmassspectrometry(TOFF_SSIMS),electrosprayionizationmassspectrometry(ESIMS).Electronparamagneticresonanceisapowerfultooltoprovetheradicalmechanismbydetectingtheradicalintermediates.Thisisnotfruitfulmethodsbecauseoftoolowradicalconcentration.3.MSAllmetal-catalyzedlivingradicalpolymerizationarenotquenchedbytheproticcompounds,suchaswater,MeOH,butclearlybyradicalscavengerssuchasDPPH,TEMPO.
4.StereochemicalstructuresThetacticityofPMMAobtainedwithvariousmetals,includingRu,Cu,Fe,Ni,PdandRhwasalmostthesame(almostatactic,slightlysyndiotactic)asthetacticityofthoseobtainedwithconventionalradicalinitiators,suchasAIBNunderthesimilarconditions.Thetriadratioofrr:mr:mmasdeterminedby13CNMRisusually58:38:4anddoesnotchangeevenwithchiraland/orbulkyligands.
NMRanalysisofmodelreactionsforpolymerizationandpolymerterminalgroupsrevealedthatthehalogenatthedormantpolymerterminalexchangewiththoseinametalcatalyst.Themixtureinsolvolysisundergoesquicklyracemizationfollowedbypolarimetry,indicatingthatthehalogenonthechiralcarbondissociatesandrecouples.NMRMacromolecules2001,34,3127-3129
TheNMRfollowedhalogen-exchangereactionAlmostimmediatelyaftermixingthetwocomponentsfollowedbyheatingtothepolymerizationtemperature,thechlorineversionof25wasclearlydetectedbyNMR.25
5.CyclicVoltammetryThecyclicvoltammetry(CV)wasusedtomeasuretheredoxpotential,becausemetalassistedlivingradicalpolymerizationistraggeredbyoxidationofthemetalcomplex,i.e.asingleelectronistransferredfromthemetaltoadormantcarbon-halogenterminal.TheredoxpotentialforRu,Fe,andCuindicatedthatacomplexwithalowerredoxpotentialinducesafastreversiblecleavageofC-Xterminal,andinturn,generatesmoreradicals.
6.Kineticsstudy(1)OverallelementaryreactionsInitiation:RX+CuBr(bpy)R.+CuBr2(bpy)R.+MRM.Propagation:RM.RMn.RMn.+CuBr2(bpy)RMnBr+CuBr(bpy)Termination:P.PkiK0Keqkp
Forawell-controlledATRPatleastkiapp=kpapp,(kiapp=kiK0,kpapp=kpKeq).Ifkiapp<>kpapp,theinitiationistoofast,alotofradicalsaregeneratedduringtheinitiationstep,irreversibleradicalterminationwillreducethetheinitiatiorefficiencyandslowthepolymerization.Theserulesalsoapplytothecross-propagationstep.ThereactivityofmonomerinATRPintermsofkpappdoesnotscalewiththetruekpvalues.Forefficientcross-propagation,kcpapp>kp.b.Itiscrucialtorapidlydeactivatethegrowingchainstoformdormantspecies.
TerminationreactionsIrreversibleterminationsoccursthroughcombinationordisproportionationpathwaysandismostsignificantatthebeginningofthepolymerization.Afterasufficientamountofthehigheroxidationstatemetalcomplexhasbeenbuiltupbytheirreversibleterminationreaction,thepersistentradicaleffectpredominatesandradicalterminationisminimized.RX+CuBr(bpy)R.+CuBr2(bpy)b.Terminationratecoefficientsdecreasesasthepolymerchainsincrease,resultedinasteadyrateofpolymerization.Thiswillhelptoformwell-definedpolymersathigherconversions.However,whenthemonomerconcentrationbecomesverylow,polymerizationslowsdownbutterminationandothersidereactionsmaystilloccurwiththeusualrate.Thusthereisacertainwindowofconcentrationsandconversionsforwell-controlledpolymerizations
Therateconstantsa.TheactivationrateconstantmeasurementmethodT:TEMPOSuppressed
TableActivationrateconstantsmeasuredat35oCNoRXComplexSkact[M-1S-1]1PEBrCuBr/2dNbpyAN0.0852MBrPCuBr/2dNbpyAN0.0523EBriBCuBr/2dNbpyAN0.604BzBrCuBr/2dNbpyAN0.0435PEBrCuBr/PMDEPAAN0.126MBrPCuBr/PMDEPAAN0.117EBriBCuBr/PMDEPAAN1.78PEClCuBr/2dNbpyAN0.000056EBriBis10timesmoreactivethanotheralkylhalides.PEBris103timesthanPECl.PMDEPAismoreactivethandNbpy.BzBrPEBrEBriBMBrP
DeactivationrateconstantmeasurementmethodSolvent:CuBr2/2dNbpykdeactinethylacetateishigherthaninacrylonitrile.kdeactofCuCl2issmallthanthatofCuBr2Ligand:CuBr2/2dNbpyisactivethanMe6TRENorPMDETAMacromolecules2001,34,430-440.
DeactivationRateconstantsTableDeactivationrateconstantmeasuredat75oCNoRadicalComplexSkdeact[M-1S-11PECuBr2/2dNbpyAN2.5x1072PECuBr2/PMDETAAN6.1x1063PECuBr2/ME6TRENAN1.4x1074PECuBr2/2dNbpyEA2.4x1085PECuCl2/2dNbpyAN4.3x106AN:AcetonitrileEA:EthylacetatePE:PhenylethyldNbpyPMDEPAMe6TREN
4-5PrecisionpolymersynthesisPendantfunctionalpolymersRadicalpolymerizationismoretolerantofpolarfunctionalitythanitsioncounterpart,leadingtodirectpolymerizationoffunctionalizedmonomersofwell-definedstructureandMWwithouttediousprotectionanddeprotectionprocess.(1)Ahydroxy-functionalmonomerHEMA(FM-1)canbepolymerizedinlivingfashionwithseveraltransitionmetalcomplexesincludingCu,Ni,Ruinbulkandinalcohol,relativelybroadMWDs(PDI=1.3=1.8).However,FM-3gavenarrowMWD(PDI=1.2-1.3)withBrEtPA/CuBr/bpy.FM-2andFM-4canundergobettercontrolledradicalpolymerization.FM-1FM-2FM-3FM-4
(2)Amino-andamido-functionalizedmonomersLivingradicalpolymerizationofFM-5wasachievedwith2-bromopropylnitrile/CuBr/bpyinDCBat50oC.FM-6canbegraftedonthesurfaceofPStparticleswithCuBr/bpy,noMWdatawasgiven.FM-8andFM-9canbepolymerizedwithcopper-basedsystem,afurtheroptimizationseemstobenecessary.FM-5FM-6FM-7FM-8FM-9
(3)IonicmonomersFM-10andFM-11canbepolymerizedwithwater-solublebromide/CuBr/bpyinaqueousmediatogivemoderatecontrolledMWsandMWDs(PDI=1.2-1.3)Epoxyandlactonegroupsremainsintactundertheconditionsformetal-catalyzedlivingradicalpolymerization.FM-16canbepolymerizedwith22/CuBr/bpyinbulkat90oCtohighconversionMWD=1.2.FM-10FM-11FM-16FM-17FM-18
(4)MacromonomersVinylacetatewasusedasaninitiatorintheATRPofSt,MacromonomerswithMn=5000,10000,15000,Mw/Mn<1.2wereobtained.Nopolymerizationofvinylacetatesegmentwasobserved.ThenmacromonomerswerecopolymeizedwithN-vinylpyrrolidioneforsynthesisofhydrogels.
2.End-functionalizedpolymersTwogeneralmethodstoprepareend-functioanlizedpolymers:a.functionalinitiatormethod;b.anend-cappingmethod.(1)Functionalinitiatormethod
Functionalinitiatorsusedinthemetal-catalyzedradicalpolymerization
a.ActivatedalkylhalidesThegeneralrulesforselectionofappropriateinitiators:(a)kiKn>kpapp,atleasttheyaresimilar(b)ThefunctionalityintheinitiatorshouldnotinterferewithATRP.(I)ForinitiatorcontainingcarboxylicacidPolymerizationswithinitiatorsormonomerscontainingcarboxylicacidsaremoredifficultbecausetheacidfunctionalitymaypoisonthecatalyst.
TheATRPofMMAusing2-bromoisobutyricacidasinitiatorLineargrowthofMWwithconversion,buthigherthanthecalculatedvalues,lowerinitiationefficiency.
(II)ForinitiatorcontaininghydroxylgroupLineargrowthofMWwithconversion;TheMWagreeswellwiththecalculatedvalues;c.NarrowMWDs;d.Highinitiationefficiency
(III)InitiatorcontainingotherfunctionalgroupsInitiatorcontainingepoxygroup
b.ActivatedsulfonylhalideswhichincludethefollowingfunctioanlitiesLinearfirst-orderkineticsplotLinearMWwithconversionquantitativeinitiationrate
Thearylsulfonylchloridesarecalledas“universalclassofATRPintiators”duetotheirhigherinitiatingrateandthenpropagationrate
(2)ω-Endfunctionalizedpolymersa.ATRPofmonomersproducedapolymerwithterminalbromineorchlorinegroup(>95%)byinsituquenchingpolymerization..b.Thehalogenatomcanbereplacedthroughavarietyofreactionsleadingtoendfunctionalpolymers.Theterminalhalogencanbereplacedbynucleophilicsubstitution,freeradicalchemistryandelectrophilicadditioncatalyzedbyLewisacids.
EC-1EC-2EC-3EC-4EC-5EC-10EC-7EC-8EC-9EC-11EC-12EC-13EC-15EC-16EC-17
c.Someexamples:(a)EC-1caneffectivelyquenchtheRu-catalyzedlivingradicalpolymerizationofMMAtogivetheω-endfunctionalizedPMMAwithaketonegroupwithhighfunctionality(Fn=1.0).Thequenchingisquantitativeandselective,andproceedsfasterwithanelectron-donatingsubstituent(X:OCH3>H>F>Clonthephenylgroupandathigherconcentration.
(b)Allyltri-n-butylstannane(c)NucleophilicsubstitutionwiththephenolateanionderivedfromEC-20.
AtomtransferradicaladditionreactionIncorporatehighreactivegroupformakingstarorothercomplicatedpolymers
(d)HalogenatomdisplacementreactionsfromhyperbranchedPSandPMAPhasetransfercatalystPartialsubstitution76%conversion85%conversion
Transformationreactionsofterminalhalogentoformfunctionalpolymers
SummaryThemethods,whichincludefunctionalinitiator,end-cappingmethods,arethemostversatileCRPtechniquetopreciselyandinexpensivelycontrolchianendfunctionalities.SynthesisofreactiveandfunctionalpolymerswithrelativelylowMW,usedinhighsolidcoatingorsolventlesscoatings.Preparationofhalide-endpolymers:usinginsynthesisofblockcopolymers,modificationoforganicandinorganicsurfaces.Halogenendgroupsmaybereplacedusingmanyorganictransformationsforsynthesisofwell-definedmono-,di-andmultifunctionaloligomerswithOH,NH2andCOOHetcforcoatings,andsegmentsforpolyesteretc.
3.BlockcopolymersBlockcopolymersviasequentialmetal-catalyzedlivingradicalpolymerization
(2)Copolymersviacombinationofother(living)
polymerizationa.AnionvinylpolymerizationThecarbanionicterminalsobtainedinlivinganionicpolymerizationcanbetransformedintocarbon-halogenbondssuitedforradicalgeneration.ThebackbonesutilizedincludePSt,polyisoprene.B-2wasusedasmacroinitiatorinATRPofanothermonomer,suchasMA,MMAandn-BuAtogiveMw/Mn=1.1-1.2
TheuseofethyleneoxideforquenchingthelivinganionicpolymerizationofstyrenefollowedbytreatmentofSOCl2resultedinacarbon-chlorineterminalforsubsequentpolymerizationof2-vinylpyridine.
b.CationicvinylpolymerizationDespitethedifferenceinactivationprocesses,someofthecarbon-halogencanbeusedasaninitiatingsitesformetal-catalyzedlivingradicalpolymerizationLivingcationicpolymerization,ingeneral,arebasedonthereversibleactivationandheterolyticdissociationofcarbon-halogenterminalsbyaLewisacid.
WhentheC-Clterminalofthepolyisobutylenecannotinitiatethelivingradicalpolymerizationduetoitslowactivityforredoxreaction,Butitcanbemodifiedintoanactiveformbyinsertingseveralunitsofstyrene.
c.RadicalvinylpolymerizationAcombinationwithconventionalpolymerizationsaffordsnovelblockcopolymersinhigheryieldsthanbefore.HalogencompoundssuchasCCl4,CHCl3,arewell-knowntelomersinconventionalradicalpolymerization.TheygiveoligomersorpolymerswithaCCl3terminal,whichcanactasaninitiatinggroupformetal-catalyzedradicalpolymerization.
Withthesamemethod,thefollowingABblockcopolymerswereprepared.
d.Ionicring-openingpolymerization(a)WiththefunctionalinitiatorsWiththesamemethod,thefollowingblockcopolymerswereprepared.
(b)ThepreparationsofABAtriblockcopolymersByterminationoflivingpoly(THF)withsodium2-bromoisopropionate
Withthesamemethod,thefollowingABAblockcopolymerswereprepared.
ABAblockcopolymerswerepreparedbymetal-catalyzedradicalpolymerizationfirst,followingCROP.
BlockcopolymerizationsoccuratthesametimeThealuminumcompoundmayhaveadualfunction,oneascatalystforanionicring-openingpolymerization,otherasanadditiveforNi-2tofacilitatethelivingradicalprocess.
e.CondensationpolymerizationCondensationpolymerizationgenerallyaffordstelechelicpolymerswithfunctionalterminals,whichcanbetransformedintoreactiveC-Clbondsforthemetal-catalyzedradicalpolymerization.
ABAblockcopolymersf.Commercialavailablepolymers
g.Inorganic/organichybrids
CommercialavailablePDMS
(3)Preparationofamphiphilicblockcopolymersa.Protectingmethod
(4)BlockefficiencyCuCl/bpy,highblockingefficiencyHighblockingefficiencymustbethatapparentrateconstantofcrosspropagationisatleastasfastasthatofthesubsequentpropagation.
Thereactivityorderforthepolymerization:
AN>MMA>St~MA1.OrderofadditionforStandMAisimportant.2.MethacrylatesandANshouldnotfollowPStandPMAblocks;Ifforsomereason,suchanorderofblockintroductionisrequired,ahalogenexchangeshouldbeused.
4.GradientcopolymersTwomethods:Automaticformationduetoaninherentmonomerreactivitydifference;Continuousadditionofsecondmonomeratacontrolledrateintothepolymerizationsystem.S=Styrene;M=MMA;Rutheniumcatalyst
4-6graftpolymersTherearethreemethodstopreparegraftpolymers:GraftfromGraftontoMacromonomermethodorgraftthrough
a.Graftfrom
b.Graftingonto
c.GraftthroughMn=5800,11900,15900;Mw/Mn<1.2AsmolecularweightofPStincreased,thegraftdensitydecreased;WhenMn=15900,noPStwasincorporatedinthecopolymers.ForthelowMWofPSt,Stcontent,MW,graftdensityincreased.Allmaterialsbehaviorshydrogelsandabsorbssignificantwater.Macromonomer
4-7PolymerbrushesThesurface-initiatedlivingradicalpolymerizationcancontrolthethicknessandthedensityofbrushes,theformerisregulatedbybrush’schainlengthandthelatterbythedensityoftheattachedinitiator.b.Thesubstratesforthesepolymerbrushesincludesiliconwafer,silicabead,silicananoparticle,poroussilicagel,silicacapillary,polymerlatex,gold.c.Andtheattachmentofpolymerbrushesalterthechemicalpropertiesoftheirsurfaceforvariousapplications.
PolymerbrushisgenerallypreparedbygraftfrommethodS-1S-2S-3S-4S-7S-6S-8S-9S-10S-11S-5
1.SphericalsilicaparticlesDynamiclightscatteringconfirmedthatthesizeofparticleshadnearlydoubled,cleavageofthecoreallowedforSECanalysisofthearms-highMWpolymerwasobtainedwithMw/Mn=1.14
2.SiliconwaferThethicknessofthepolymerlayerincreasedupto70nmindirectproportiontotheMWsoffreepolymersproducedinsolution,althoughtheMWsofthegraftpolymerswereunknown.Thegraftdensitycanbevariedinawiderangebetween0.07-0.7chains/nm2byphotodecomposingthesurface-fixedinitiator,wherethehighestvalueisatleast10timeslargerthanthoseofthepolymerbrushespreparedbytheabsorptionofblockcopolymeronsurface.
AmphiphilicblockcopolymersWatercontactanglewasdecreasedfrom86oto18o
3.GoldtBA,MMA,isobornylmethacrylate,2-(N,N-dimethylamino)ethylmethacrylate,andHEMAwerepolymerizedfromS-6withcoppercatalyststoformlayersrangingfrom5to48nmwithvaryingcontactanglesdependingonthepolymers.Theinitiatorcanbeattachedalsoonpatternedareabymicroprintingmethod.Thehydrophobicpolymersasbrushesareresistanttoetchants,whichpermitstheselectiveetchingofgoldsurface.S-6S-7
4-8HyperbranchedpolymersInvinylpolymerization,hyperbranchedpolymerscanbeobtainedfromthemonomersthathaveaninitiatinggroupalongwithvinylgroup,thispolymerizationiscalledasself-condensationvinylpolymerization(SCVP).
4-9Starpolymers
4-10Outlook
Theadvantagesofthemetal-catalyzedradicalpolymerizationVersatilitytowardsavarietyofmonomersFeasibilityinawiderangeofreactionconditionsTherelativelyeasyaccesstothematerialsThekineticsandmechanismsofpolymerizationsThenatureofthetruegrowingintermediatesthatareconsideredtoassociatewiththemetalcatalyst.Anumberofinterestingandchallengingproblemsthatarestillwaitingforsolutionsandanswers:
Aretheyreallyradicalsidenticalorsimilartothoseinclassicalfreeradicalpolymerizations?3.Theguidingprinciplesfordesigningmetalcatalysts,andthenovelpolymersthatcanbesynthesizedbymetal-catalyzedradicalpolymerizationbutcannotbebyotherlivingradicalpolymerizations.4.Developmentofmoreefficientandversatilecatalystsystemsforvariousmonomersincludingnonconjugatedvinylmonomers,aswellasthesynthesisofspecificpolymerswithspecialarchitechturesandtheapplicationtoindustryprocess.
FutureresearchinATRPwillbefocusedontheareasConstructionofastructure-reactivitycorrelationforallcomponentsofanATRPdevelopmentofnew,moreefficient,moreselective,lessexpensive,andenvironmentallysoundATRPcatalyticsystem.BuildingarelationshipbetweenmolecularstructureandmacroscopicpropertiesformaterialsmadebyATRP.Reducetheamountofcatalyst(ppm).
ProposedmechanismfortheAGETATRPAGETATRPProposedmechanismfortheSR&NIprocessinATRPJ.Am.Chem.Soc.;127(11),3825-3830(2005).
SET-LRPSingle-electron-transfer(SET)MechanismofSET-LRPJ.Am.Chem.Soc.2006,128,14156-14165.
FeaturesofSET-LRPMonomer:MA,MMAandVCRoomtemperatureCatalyst:verysmallamountUltrafastMolecularweight:1.5x106Moleculardistribution:NarrowSolvent:Dipolaraprotic
“希望工程”义演制作:王爱群
某文艺团体为“希望工程”募捐组织了一场义演,共售出1000张票,筹得票款6950元,成人票与学生票各售出多少张?(已知成人票8元,学生票5元每张)
设售出的学生票为x张,填写下表学生成人票数/张票款/元x1000-x5x8(1000-x)
设所得的学生票款为y元,填写下表学生成人票数/张票款/元y6950-yy56950-y8
想一想如果票价不变,那么售出1000张票所得票款可能是6930元?学生票、成人票各是多少张呢?为什么?
随堂练习小明用172元钱买了两种书,共10本,单价分别为18元、10元,每种书小明各买了多少本?
大诗人:李白
趣味数学:李白街上走,提壶去买酒;遇店加一倍,见花喝一斗;三遇店和花,喝完壶中酒;试问酒壶中,原有多少酒?请同学们列表分析题中的等量关系'
您可能关注的文档
- 最新[最新]正能量读后感课件PPT.ppt
- 最新[最新]第十五章 眼的屈光和调节 第一节 正常屈光状态和调节课件PPT.ppt
- 最新[最新]眼睑 泪器课件PPT.ppt
- 最新[最新]目力保健专题常识讲座课件PPT.ppt
- 最新[最新]轧制过程中的力学概述课件PPT.ppt
- 最新[材料科学]磁痕分析课件PPT.ppt
- 最新[材料科学]复习重点课件PPT.ppt
- 最新[汇总]第十一章 幼儿的心理健康及其评价课件PPT.ppt
- 最新[汇总]第八讲 汇编语言程序的阅读与理解111201课件PPT.ppt
- 最新[理学]影响亲核取代反应活性的因素课件PPT.ppt
- 最新[理学]第1讲-数学建模简介课件PPT.ppt
- 最新[理学]菲涅耳公式与半波损失课件PPT课件.ppt
- 最新[理学]第二章、行列式课件PPT.ppt
- 最新[理学]第5讲 实验2 二极管稳压电路课件PPT.ppt
- 最新[生物学]7生物素-亲和素免疫放大技术课件PPT.ppt
- 最新[畜牧兽医]水产动物营养和饲料学3-水产动物饲料营养组成课件PPT.ppt
- 最新[研究生入学考试]四川大学化工原理下册重点复习课件PPT.ppt
- 最新[研究生入学考试]南京理工大学 数字电路课件课件PPT.ppt